Elizabeth Baxter - Graduate Student
ebaxter (at) ucsd.edu
M.S. University of California, San Diego - 2010
B.S. Univeristy of California, Santa Cruz - 2006

Research Description:
While evolution selects for robust folders, it must do this while conserving and selecting for function. This competition between selection for efficient folders and function can introduce frustration and prevent a landscape from becoming perfectly funneled. It has been observed that functional regions of many proteins do not aid folding and may in fact interfere with it. It may be possible then to use frustration in folding as an assay for functional regions.
My research uses a combined theoretical and experimental approach to characterize the relationship between folding and function in the recently discovered diabetes drug target, mitoNEET. mitoNEET contains a 2Fe-2S cluster, and belongs to a previously uncharacterized ancient family of proteins for which the hallmark is the presence of a unique 39 amino acid CDGSH domain. I use structure-based simulations as a predictive tool to guide experimental studies with mitoNEET. Using structure-based simulations I characterize the folding and functional landscape of mitoNEET and identify regions that slow down folding and regulate conformational dynamics. Experimentally I perturb these regions with mutagenesis and look for changes in the biophysical properties of the metal center.
Publications:
Baxter, E. L.; Jennings, P. A.; Onuchic, J. N., Strand swapping regulates the iron-sulfur cluster in the diabetes drug target mitoNEET. Proceedings of the National Academy of Sciences of the United States of America 2012, 109 (6), 1955-1960; (Press release)
Baxter, E. L.; Jennings, P. A.; Onuchic, J. N., Interdomain communication revealed in the diabetes drug target mitoNEET. Proceedings of the National Academy of Sciences of the United States of America 2011, 108 (13), 5266-5271;
Figures:
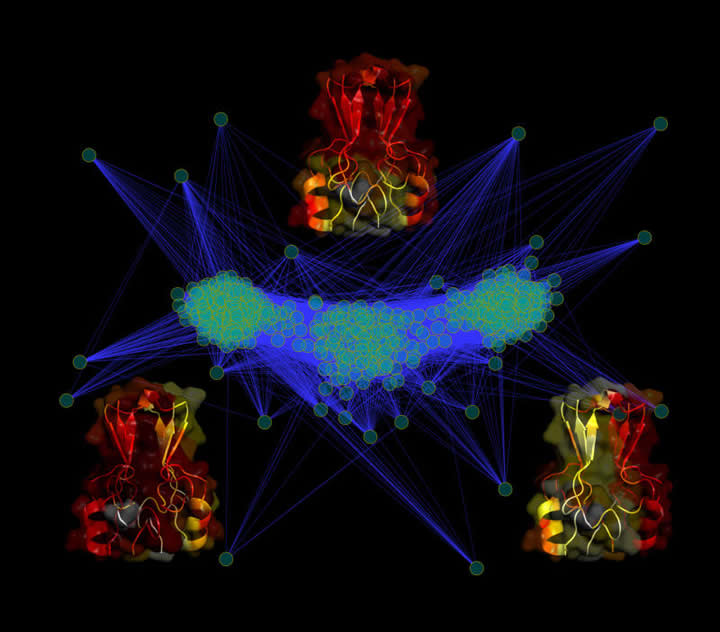
Cluster analysis of the transition state in MitoNEET reveal an alternate route in folding. This type of frustration in the folding landscape provides us with clues about functional dynamics.
